What are antibiotics?
Antibiotics were first discovered in 1928 by a scientist named Sir Alexander Fleming. Fleming discovered that when Penicillium notatum, a fungus, grew on a culture plate also growing Staphylococcus, it prevented growth of the bacteria. Upon further investigation, Fleming realized that the lack of bacterial growth was a direct result of the antibiotic properties of Penicillium notatum. Later, scientists would isolate this fungus and create the first utilized antibiotic, calling it Penicillin. The history behind the story of the development of Penicillin is in fact quite interesting, and can be read here.
Although Sir Alexander Fleming is credited for observing arguably one of the largest scientific breakthroughs in medicine, he was not the first to observe the properties of antibacterial substances. History shows that the use of fungus and other bacteria were used to treat diseases long before 1928.
As Fleming discovered, antibiotics are naturally occurring and work by inhibiting growth of bacteria, or by killing them directly. Most antibiotics work by inhibiting important cellular components needed for the growth and reproduction of bacterial cells. For example, some antibiotics prohibit the formation of peptidoglycan, an important component of bacterial cell walls that protect them from the outside world. By inhibiting the growth of bacteria, antibiotics are able to kill off some of the bacteria, and therefore allow the immune system a chance to fight back against the unwelcome bacteria.
What is antibiotic resistance and why is it important?

Since antibiotics were first discovered in the 1940’s, antibiotic resistance has become increasingly common. According to FDA, “antibiotic resistance is one of the world’s most pressing public health problems today.” Antibiotic resistance is due in large part the the overprescription of antibiotics, as well as the incorrect dosage of antibiotics. Through the overuse of antibiotics and antimicrobials, many bacterial strains have developed/acquired antibiotic resistant genes that allow them survive even when antibiotics are used.
How does the overprescription of antibiotics cause antibiotic resistance? When you take an antibiotic it kills both the good and the bad bacteria inside your body. If antibiotic resistant strains are present, the antibiotics will be ineffective against those cells. Since the antibiotics kill most of the other bacteria cells that compete with antibiotic resistant strains, the resistant cells no longer have competition for resources, and are able to take over and multiply.
Antibiotic resistance is a scary reality, but there are things that can be done to help slow the advancement of antibiotic resistance. Below is a list of things you can do to help combat antibiotic resistance:
- Finish your Prescription: finishing the full course of the antibiotic ensure that all of the pathogenic bacteria die. DO NOT save your antibiotic for further use.
- Question your Doctor: make sure your doctor has confirmed you have a bacterial infection before you get your prescription for an antibiotic. Taking an antibiotic when not needed has been a major cause of antibiotic resistance, and often allows bad strains to take advantage of a lack of competition.
- Ask for a Specific Antibiotic: ask for your doctor for an antibiotic that targets the specific bacteria that you are infected with. By using a more specific antibiotic, a smaller number of bacteria are targeted, which allow good bacteria to keep the bad bacteria in check.
How does bacteria become antibiotic resistant?
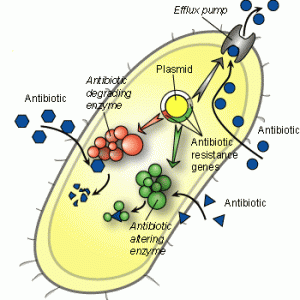
The problem of antibiotic resistance is huge in scope. When antibiotics are given to a person or animal to treat a bacterial infection, that antibiotic puts a huge amount of pressure onto the bacteria. This pressure causes the bacteria to change as fast as it can to try and get around the antibiotic. Changes are mostly random, but if one bacterium gets lucky enough and develops some way to fight the antibiotic, that bacterium would become dominant in relation to the other bacteria. Eventually, the entire infection will be composed of antibiotic resistant ancestors of that first lucky bacterium. The question is, how can a bacterium fight an antibiotic?
It turns out, bacteria fight antibiotics in three main ways. The first way is the most straightforward, because the bacteria just destroy the antibiotic before it can do its job. This method is very effective for the bacteria, but is difficult to pull off because antibiotics are often given in cocktails. That is, multiple compounds are used at once to fight a single infection. The chances that a bacteria could break down all of the compounds is very rare.
The second method that bacteria use to fight antibiotics is by changing the antibiotic in some way. Imagine that the antibiotic is a stick of dynamite with a lit fuse. The bacteria could just yank off the fuse before the dynamite explodes, making the dynamite harmless. Like the first method, this one is tough for the bacteria to pull off because of the variety of antibiotics used at once. The third method of antibiotic resistance is the development of efflux pumps talked about in the next section.
Efflux Pumps… a common method of developing antibiotic resistance
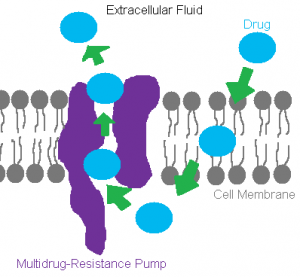
Among the many mechanisms that result in acquiring antibiotic resistance, efflux pumps are one of the more widely utilized mechanisms bacteria use to develop resistance towards the antibiotics used to wipe them out. What is an efflux pump? As mentioned earlier, efflux pumps are a type of transport system used by bacteria to remove or “efflux” a certain molecule that happens to get into the cell.
With the rising concern of an antibiotic “apocalypse”, the research going into deciphering the mechanisms used to achieve resistance is at an all time high. Of course with growing research comes diverse opinions, and the hot topic of efflux pumps have sparked some debate within the field. Scientists argue that new evidence proves efflux pumps have both a physiological function within the cell in addition to the evolutionary acquired function of overcoming the antibiotic era. Regardless of the true indented functionality of efflux pumps, bacteria have been able to coalesce these activities to conquer the effectiveness of antibiotics–establishing research on these pumps more critical.
The mechanism of a common efflux pump is summarized in the figure above. An antibiotic enters the cell through a channel in the membrane, is targeted by the cell as harmful, and then is ushered out of the cell through an efflux pump with the help of protein transporters and energy from the cell. Still don’t understand how an efflux pump works? Check out this cool video!
As with all other aspects of a cell, specific genes are responsible for building and maintaining these pumps. The genes contributing to efflux pumps who yield pathways for multi-drug resistances have become an important target in research, as finding a way to exploit these pumps against the cell could potentially be a large stride in the fight against antibiotic resistance.
What does all of this mean?
Antibiotic resistance has long been overlooked, but it is a real threat that is growing by the day. Now is the time for a new generation of antibiotics. Currently, little effort is being put in to develop new antibiotics in response to resistance. A new generation of antibiotics has not been produced in nearly 20 years. The longer we fail to develop new antibiotics, the fewer drugs we can count on to be effective. In fact, very few drugs today are resistant-free, most have had resistance building up for years. By remaining ignorant to the danger of antibiotic resistance, the drugs we depend on for our health will over time become ineffective. Imagine not having antibiotics to cure basic infections like pink eye or bronchitis. Even a simple infection from a papercut could have drastic effects without antibiotics. This is the path we are on and we must take action. By taking simple measures like finishing our prescription and advocating the development of new antibiotics, we can minimize this threat and advance effective antibiotics to future generations.
Written by: Daniel Stroud, Maggie Kraemer, Dan Franzen, and Kaitlin Burdick
I have heard that most mutations that make bacteria antibiotic resistant are disadvantageous if there are no antibiotics present, meaning the resistance should evolve away if given time to. Do you know anything about that?
How about antibiotics being fed to meat animals like cattle and chickens? How does that effect the picture?
“This pressure causes the bacteria to change as fast as it can to try and get around the antibiotic. Changes are mostly random…” This line makes it sound like the antibiotics are making mutate faster, likely you are just referring to strong selection pressure making certain mutations multiply faster, but it made me curious as to whether some antibiotics do actually cause higher mutation rates?
Thanks Christina!
Here are a few answers to your questions.
1. Antibiotic resistances ARE disadvantageous when the antibiotic is not present, you are correct. This is because the mechanisms for resistance usually cost the cell a lot of energy to undergo. Things like efflux pumps require ATP to function. It would be expected for a bacterium to be less fit after antibiotics are removed from the environment, however the antibiotics have killed off most of the non-resistant competition. Therefore, the resistant bacteria become the most fit bacteria in the population. In cases like this, overall fitness is less important than relative fitness.
2. Antibiotics being fed to meat animals is a huge issue. An excellent resource would be the CDC at this link: http://www.cdc.gov/narms/faq.html. Basically, antibiotic use in livestock produces antibiotic resistant bacteria just like in humans. Then, the antibiotic resistant bacteria can enter the food we eat via contamination (usually fecal).
3. For the pressure, you are right that we are referring to selection pressure. A strong selection pressure for the antibiotic resistance allows for that mutation to spread rapidly through a population. As far as we know, antibiotics do not cause higher mutation rates.
I found this to be very helpful, especially the ways that we can try to prevent antibiotic resistance. I was also really interested in the ways that bacteria become resistant because I had never known that before. Do you know any specifics of how scientists are trying to exploit these efflux pumps?
Thanks for the comment!
The goal for targeting efflux pumps would be for scientists to identify a target that is unique to bacteria or any sort of pathogen. Since humans have a variety of pumps that are very similar to efflux pumps, any sort of target for bacteria could potentially also target humans. Once a target is identified, scientists would look for some sort of inhibitor for that component of the pump. This could be anything from a compound that binds that component to a transcriptional inhibitor of a gene upstream of the target. Anything that interrupts the component could work.
Once an inhibitor for the target component is made or found, it is possible that the efflux pump the inhibitor is designed to disable could actually pump out the inhibitor before the inhibitor does anything. It is really a molecular arms race between science and bacteria.
This is appears to be a very important topic regarding the safety of everyone. From your blog post, it appears that there are some things that people can do on their own to help stop the spread of antibiotic resistance. However, it appears that the next step is mostly the responsibility of scientists to get a step ahead and create new effective antibiotics that bacteria have not yet adapted to overcome. What do you guys think will cause scientists to shift and take on this task? Does the general public need to become more aware of the situation, and then do they need to tell scientists that this is a priority? Or do you think this is something that scientists would like to investigate, but they need the public to become more educated so that they can receive funding to do more research?
Interesting questions, John.
The bulk of the work to be done to combat antibiotic resistance will be done by scientists, that is correct. The role of the everyday citizen is to help increase the lifespan of our current antibiotics, not to generate new ways to fight disease. To do this, citizens need to be aware of how antibiotics work, how to take antibiotics, and if they even need antibiotics in the first place.
Current research into new antibiotics is a losing battle for most pharmaceutical companies, in fact most pharmaceutical companies (15 of the largest 18) have stopped developing new antibiotics. The reason this is happening is multi-faceted.
1. Antibiotics are relatively cheap. Big drug companies don’t turn a very large profit in antibiotics compared to other more expensive drugs.
2. Lack of funding from both the federal and private sectors. For the same reasons as #1, there is less funding available. Additionally, economic instability in recent years has caused money to be funneled out of the money losing field of antibiotic development.
3. Antibiotics are uncertain. Even when a great antibiotic comes out, chances are it would eventually become resisted to by bacteria, causing the antibiotic to become obsolete. Something like insulin or cholesterol medication will never become obsolete.
The information here is found in this article: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/
This blog post is really informative . I like how you guys started out by providing the background information about antibiotics and from there ,build up the awareness about the antibiotic resistant. This give me insight on how antibiotic resistant developed and ways to prevent that in small to large scopes.
This information was very informative and was also easy to read. Horizontal gene transfer is big with the movement of genetic material. Is there an effective way to prohibit this to stop the cell from passing on resistance genes?
I think that the links and the video at the end of the post really pulls the whole thing together. It helps with people who are on different levels of understanding and makes it easy to read whether you know the material or not.
Being a Microbiology major this is very pertinent to what I am learning. I think the info was laid out nicely and easily understood. In your opinion what would you say the most pressing matter, as far as antibiotic resistance goes, is at the moment?
In my mind, the most pressing matter would be the reemergence of diseases that we thought were cured. For example, Gonorrhea has become increasingly antibiotic resistant. The drugs needed to treat this disease constantly change, and soon our repertoire of drugs will run out. At this point in time, the CDC recommends a dual treatment composed of a single injection in conjunction with oral antibiotics.
Its scary to think about simple bacterial infections becoming untreatable, but that is the trend.
Hey, I really like the way you laid the issue out– made it easy to understand how and why resistance occurs. Have you researched any of the new approaches scientists are discovering to combat antibiotic resistance? Some are really novel, and quite fascinating to read about.
I agree that antibiotic resistance is a very important topic! As time goes on it is going to be more prominent in our world and it is important to advocate to the public. This article does a great job of physically showing how quickly mutations can occur: http://news.harvard.edu/gazette/story/2016/09/a-cinematic-approach-to-drug-resistance/
Give it a read and watch the video if you have a chance!
This post is very informative and goes into detail about a very important topic. Antibiotic resistance is a growing problem, and if it continues with no new ways to treat bacteria we could be in trouble. Do you know if there are any alternatives currently being looked at besides antibiotics as a way to treat/kill bacteria?
I know that a lot of research is going into biomaterial based antibiotics. The idea is that an antibiotic needs to disrupt something metabolically important to the bacteria, that is also unique to the bacteria. The disruption doesn’t necessarily need to be a traditional drug.
An example of a possible antibiotic biomaterial is some sort of synthetic compound that could enter bacterial cells and coalesce and kill the cell. The difference in human cell vs bacterial cell size could be utilized here. Larger human cells could tolerate the compound whereas bacterial cells might not be able to. This situation is hypothetical, but realistic.
This is a really important topic! I think you did a really great job of explaining antibiotic resistance and defining important terms. Have you found anything in your research about how antibiotics in the food industry will affect resistance?
Antibiotics in the food industry is a huge issue. Large scale usage of antibiotics in livestock and crops is extremely damaging to our arsenal of usable antibiotics.
Animals raised on antibiotics grow better, and stay healthier than animals that are not raised on antibiotics. It is difficult to tell farmers to opt to have smaller animals when we also have a global food shortage.
On top of animal antibiotics, crops are often sprayed down with antibiotics. Normally slow growing and expensive crops like citrus, apples, and other fruits are targeted most often by antibiotics. In fact, the citrus industry in Florida is appealing to the FDA to spray millions of pounds of antibiotics onto the citrus crops to help delay a citrus disease that is spreading.
I’ve come across this issue multiple times, but have never really read much into the danger of antibiotic resistance. Very interesting! The basic idea of bacteria becoming resistant to the meds we constantly expose them to is fairly intuitive. However, the extent of the threat to human health as a whole seems to be something that people know little, if anything, about. How long do you think it’ll take before a large portion (say, 40-50%) of commonly prescribed antibiotics will become more or less ineffective?
Very well written article that follows an adequately scientific approach yet is general enough for the majority of the general public to read, understand, and not become overloaded with jargon and unnecessary info.